What is a Supercritical Fluid?
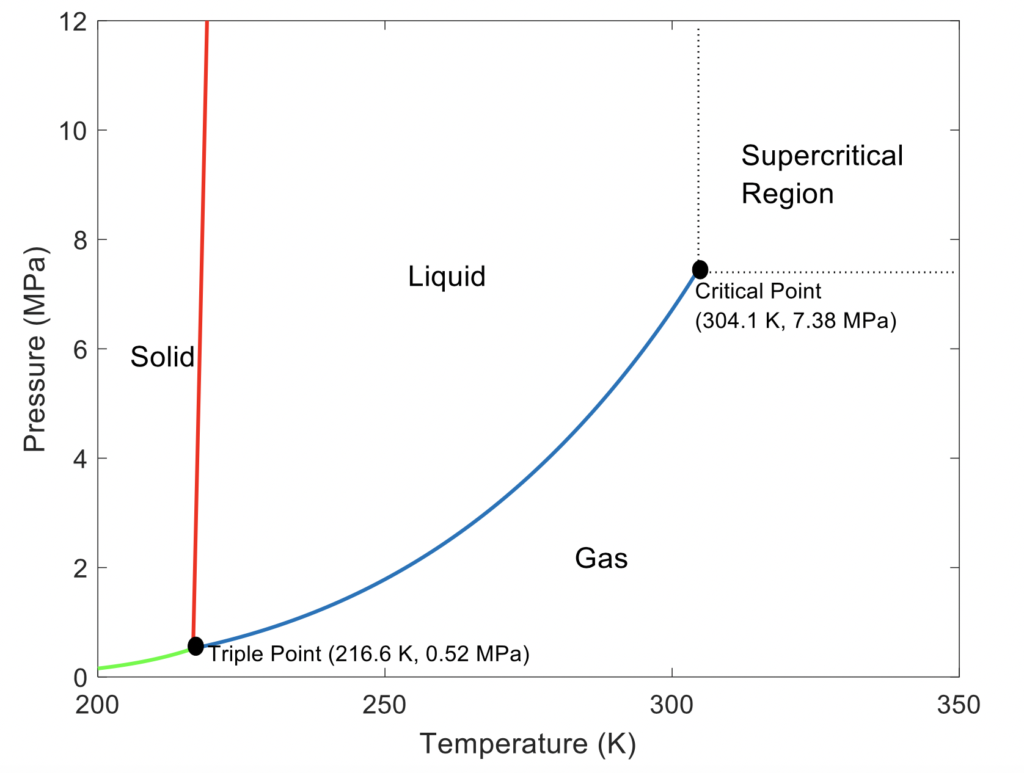
Supercritical Fluids (SCFs) are compounds at a temperature and pressure beyond their critical point. Carbon dioxide is the most widely used SCF owing to its reasonable critical point (304.1 K and 7.38 MPa), low cost, inert and non-toxic character.
SCFs are a single phase with properties that lie between the properties of typical gases and that of typical liquids. Gaseous carbon dioxide (at 298 K and 1 atm) has a density of 1.8 kg/m3. Liquid carbon dioxide (at 298 K and 10 MPa) has a density of 818.88 kg/m3. Supercritical Carbon Dioxide’s density can be tuned (at 350 K and 8 MPa it is 164 kg/m3 while at 305 K and 30 MPa it is 941 kg/m3). This density shift over narrow temperature and pressure changes is key to many of the opportunities associated with SCF applications.
SCF property tunability extends well beyond density. Density, enthalpy, entropy, diffusivity, viscosity, solubility and more depend on the selected SCF and on the operating conditions of temperature and pressure. This tunability presents an opportunity for the development of a wide range of applications.
Carbon Dioxide Phase Diagram